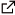Accueil du site > Équipes > Arômes Parfums Synthèse Modélisation > Thème Synthèse et méthodologies catalytiques
Thème Synthèse et méthodologies catalytiques
- Présentation
- Composition
- Recherche
- Publications et Brevets depuis 2003
- Equipement
- Collaborations
- Contact
Publications et Brevets depuis 2003
![]() 2017_______________________________________________
2017_______________________________________________
- Synthesis and olfactory evaluation of spiro tricyclic diether structures P. Ondet, C. Plessis, G. Lemière, E. Duñach, Flavour & Fragrance J., 2017, 32, 119-125.
- Cyclisations Catalysed by Bismuth(III) Triflate P. Ondet, G. Lemière, E. Duñach, Revue, Eur. J. Org. Chem., 2017, 761-780.
- Friedel-Crafts acylation with a polymer supported indium(III) catalyst V. Morizur, D. Hector, S. Olivero, J. R. Desmurs, E. Duñach, Synfacts, 2016, 10, 1105. DOI : 10.1055/s-0036-1589223.
- Bond Strength and Reactivity Scales for Lewis Superacid Adducts : A Comparative Study with In(OTf)3 and Al(OTf)3 G. Compain, L. Sick, L. Massi, E. Duñach, J. F. Gal, ChemPhysChem., 2017, 18, 683-691.
- Electrosynthesis of boronic acids and esters S. Olivero, E. Duñach, revue, Current Opinion in Electrochemistry, 2017, 2, 38-42.
- Quantitative ligand affinity scales for metal triflate salts. Application to isomer differentiation C. Iacobucci, N. Jouini, L. Massi, S. Olivero, G. F. De Angelis, E. Duñach, J.-F. Gal, PlusPlusChem, 2017, 82, 498-506. DOI : 10.1002/cplu201700124
- Electrochemical cyclizations of organic halides catalyzed by electrogenerated nickel(I) complexes : towards environmentally friendly methodologies M. J. Medeiros, S. Olivero, E. Duñach, revue, Electrochim. Acta, 2017, 373-381.
- Atom Economical Catalytic Direct Substitution of N,O-Acetals with Simple Ketones V. Dalla, S. Antoniotti, E. Dunach, Eur. J. Org. Chem., 2017, 4445-4460.
- Tuning the Reactivity of Functionalized Diallylic Alcohols : Brønsted Acid vs. Lewis Acid Catalysis L. Lempenauer, E. Duñach, G. Lemière, Chemistry, Eur. J., 2017, 23, 10285-10288.
![]() 2016_______________________________________________
2016_______________________________________________
- Enolizable Carbonyls and N,O-Acetals : A Rational Approach for Room-Temperature Lewis Superacid-Catalyzed Direct α-Amidoalkylation of Ketones and Aldehydes B. Touati, A. El Bouakher, C. Taillier, R. Ben Othman, M. Trabelsi-Ayadi, S. Antoniotti, E. Duñach, V. Dalla, Chem. Eur J. 2016, 22, 6012-6022
- Ionic Liquids for the Electroreductive Radical Cyclization of Unsaturated Bromo Derivatives Catalyzed by Nickel(II) complexes M. J. Neto, E. Duñach, J. M. S. S. Esperança, A. P. Esteves, M. J. Medeiros, M. M. Silva, J. Electrochem. Soc 2016 2016, 163, G21-G25.
- Ionic Liquids for the Electroreductive Radical Cyclization of Unsaturated Bromo Derivatives Catalyzed by Nickel(II) complexes M. J. Neto, E. Duñach, J. M. S. S. Esperança, A. P. Esteves, M. J. Medeiros, M. M. Silva, J. Electrochem. Soc 2016 2016, 163, G21-G25.
- Catalytic rearrangement of 2-alkoxy diallyl alcohols : a straightforward access to polysubstituted cyclopentenones L. Lempenauer, E. Duñach, G. Lemière, Org. Lett., 2016, 18, 1326-1329.
- Novel metal sulfonate polymers as catalysts for the heterogeneous acylation of aromatic derivatives V. Morizur, D. Hector, S. Olivero, J. R. Desmurs, E. Duñach, Eur. J. Org. Chem., 2016, 3126-3129.
- Single ion solid aromatic polymer electrolytes with high mechanical resistance for Lithium metal batteries V. Morizur, M. Braglia, S. Olivero, J. R. Desmurs, P. Knauth, E. Duñach, New J. Chem., 2016, 3126-3129. DOI : 10.1039/C6NJ00670A
- Bi(III) triflate catalysed tandem cyclisations towards complex polycyclic ethers P. Ondet, L. Lempenauer, E. Duñach, G. Lemière, Org. Chem. Frontiers, 2016, 3, 999-1003.
- Acid-Catalyzed Synthesis of Thiazolidin-4-ones N. Sadou, S. Aichouche-Bouzroura, R. Nechak, B. Nedjar-Kolli, V. Morizur, S. Poulain-Martini, E. Dunach, Polycyclic Aromatic Compounds, 2016, 1-11.
![]() 2015_______________________________________________
2015_______________________________________________
- Catalytic carbonyl-ene reaction with ketones : evidence for a retro-ene process P. Tremel, C. Iacobucci, L. Massi, S. Olivero, J. F. Gal, E. Duñach, New. J. Chem. 2015, 39, 7453-7458. link
- Catalysis of the acylation of aromatic derivatives by metallic tosylates V. Morizur, J. Szafranek, D. Bonhomme, S. Olivero, J. R. Desmurs, E. Duñach, Tetrahedron 2015, 71, 1752-1763. link
- Synthesis and odour evaluation of allenic derivatives I. Diaf, A. Joffrin, G. Lemière, E. Dunach, Flavour & Fragrance J. 2015, 478-484. link
- Electrosynthèse d’acides et d’esters boroniques S. Olivero, E. Duñach, L’Actualité Chimique 2015, 400-401, 29-30.
- Cycloisomerization of Allene–Enol Ethers under Bi(OTf)3 Catalysis Ondet, P. ; Joffrin, A. ; Diaf, I. ; Lemiere, G. ; Dunach, E. Org. Lett. 2015, 17, 1002-1005. link
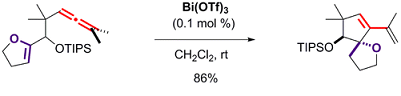
- Synthesis and odour evaluation of tricyclic ether derivatives containing a cis-1,2-dimethyl norbornane moiety Clinet, A. ; Chaignaud, M. ; Clinet, J.-C. ; Duñach, E. Flavour & Fragrance J. 2014, Flavour & Fragrance J. 2015, 30, 165-170. link
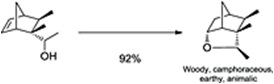
- Synthesis and antimicrobial evaluation of novel 4-thiazolodinones containing a pyrone moiety R. Nechak, S. Aichouche-Bouzroura, Y. Benmaleek, L. Salhi, S. Poulain-Martini, V. Morizur, E. Dunach, B. Nedjar-Kolli, Synth. Commun. 2015, 45, 262-272. link
![]() 2014_______________________________________________
2014_______________________________________________
- Novel lithium and sodium salts of sulfonamides and bis(sulfonyl)imides : synthesis and electrical conductivity Morizur, V. ; Olivero, S. ; Desmurs, J. R. ; Knauth, P. ; Duñach, E. New J. Chem. 2014, 38, 6193-6197. link
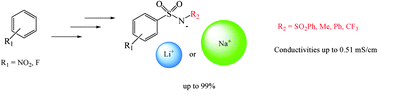
- Access to polycyclic derivatives via triflate-catalyzed intramolecular hydroarylation Cacciuttolo, B. ; Poulain-Martini, S. ; Fontane-Vive, F. ; Hussein Abdou, M. A. ; El Kashef, H. ; Duñach, E. Eur J. Org. Chem. 2014, 7458-7468.link
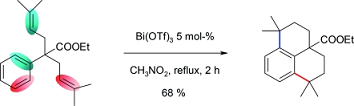
- Carbon-carbon bond formation by Lewis superacid catalysis Dunach, E. Chemistry & Biodiversity 2014, 11, 1752-1763.
link
- Bi(III)-catalysed synthesis of substituted indanes by a double hydroarylation of unactivated 1,3-dienes Cacciuttolo, B. ; Ondet, P. ; Poulain-Martini, S. ; Lemiere, G. ; Dunach, E. Org. Chem. Frontiers 2014, 1, 765-769. link
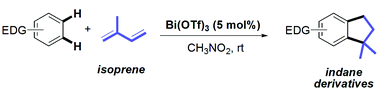
- Application of cooperative iron/copper catalysis to a palladium-free borylation of aryl bromides with pinacolborane Labre, F. ; Gimbert, Y. ; Bannwarth, P. ; Olivero, S. ; Duñach, E. ; Chavant, P-Y. Org. Lett. 2014, 16, 2366-2369. link

- Electrochemical catalytic cyclization reactions using environmentally friendly methodologies Dunach, E. ; Medeiros, M. J. ; Olivero, S. Renewable Energy Global Innovations Series 2014, ISSN 2291-2460.
- Catàlisi de la funcionalitzacio d’oléfines per superàcids de Lewis Dunach, E. Societat Catalana de Quimica 2013, 12, 50-54. ISSN : 2013-9853.
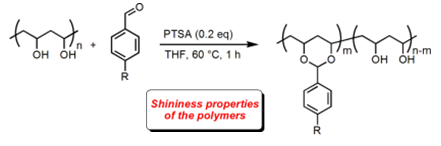
- Poly(vinyl alcohol) Functionalization with Aldehydes in Organic Solvents : Shining Properties of Poly(vinyl acetals)
Delattre, E. ; Lemière, G. ; Boulay, B. ; Desmurs, J.R. ; Dunach, E. J. Appl. Polym. Sci. 2014, 131, 40677. link
- Metal Triflate-Catalysed Synthesis of Polycyclic Tertiary Alcohols by Cyclisation of gamma-Allenic Ketones
Diaf, I. ; Lemière, G. ; Dunach, E. Angew. Chem., Int. Ed. 2014 , 53, 4177-4180. link
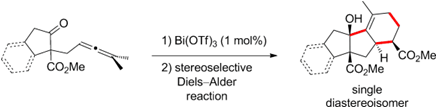
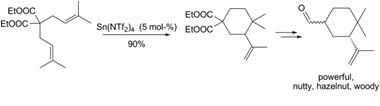
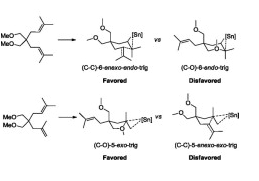
- Mechanistic insights into the 1,6-diene cycloisomerization catalyzed by Sn(NTf2)4 Nava, P. ; Carissian, Y. ; Drujon, J. ; Grau, F. ; Godeau, J. ; Antoniotti, S. ; Duñach, E. ; Humbel, S. ChemCatChem 2014, 2, 663-672. link

- Preparation and olfactory evaluation of mono- and bicyclic compounds featuring gem-dimethylcyclohexane structures Godeau, J. ; Grau, F. ; Antoniotti, S. ; Duñach, E. Flavour & Fragrance J. 2014, 29, 59-66. link
![]() 2013_______________________________________________
2013_______________________________________________
- Electrochemical catalytic cyclization reactions using environmentally friendly methodologies. Dunach, E. ; Medeiros, M. J. ; Olivero, S. J. Electrochem. Soc., 2013, 160, 3112-3116. link
- Essential oil composition and antibacterial activity of the different parts of Thymus maroccanus Ball : An Endemic Species in Morocco. Romane, A. ; Belaqziz, R. ; Bahri, F. ; Fernandez, X. ; Antoniotti, S. ; Duñach, E. Natural Product Research, 2013, 27, 1700-1704. link
- Indirect Electrochemical Cyclization of Bromoalkoxylated Derivatives using Environmentally Friendly Methodologies Duñach, E. ; Medeiros, M.J. ; Olivero, S. ECS Transactions, 2013, 45, 9-13.
- Catalyse et synthèse asymétrique Constantieux, T. ; Duñach, E. L’Actualité Chimique, 2013, 377, 14-17.
- An efficient conversion of maleimide derivatives to 2-thioxo imidazolidinones Salhi, L. ; Bouzroura-Aichouche, S. ; Benmaleek, Y. ;Bentarzi, Y. ; Poulain-Martini, S. ; Cacciuttolo, B. ; Dunach, E. ; Nedjar-Kolli, B. Org. Commun. 2013, 6, 87-94.
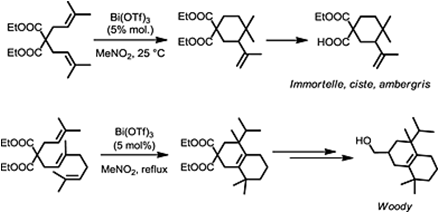
- Catalytic Activation of Olefins by Metal Triflates and Triflimides : Application to Fragrance Chemistry.
Lemière, G. ; Dunach, E. Chem. Eur. J. 2013, 19, 3270-3280. link
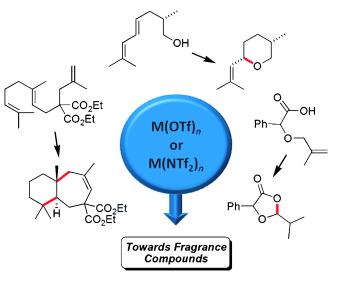
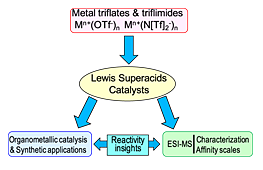
- Metal triflates and triflimides as Lewis superacids : preparation, synthetic application and affinity tests by mass spectrometry. Gal, J. F. ; Iacobucci, C. ; Monfardini, I. ; Massi, L. ; Duñach, E. ; Olivero, S. ; (Mini-review), J. Phys. Org. Chem., 2013, 26, 87-97. link
- Synthesis and odour evaluation of alcohols bearing a cis-1,2-dimethyl norbornane moiety. Clinet, A. ; Chaignaud, M. ; Clinet, J.-C. ; Dunach, E. Flavour Fragrance J. 2013, 28, 53-61
link
![]() 2012_______________________________________________
2012_______________________________________________
- Experimental and Theoretical Studies on the Bismuth-Triflate-Catalysed Cycloisomerisation of 1,6,10-Trienes and Aryl Polyenes.
Godeau, J. ; Fontaine-Vive, F. ; Antoniotti, S. ; Dunach, E. Chem. Eur. J. 2012, 18, 16815-16822 link
- Bi(OTf)3-Catalyzed Cycloisomerization of Aryl-Allenes.
Lemiere, G. ; Cacciuttolo, B. ; Belhassen, E. ; Dunach, E. Org. Lett. 2012, 14 , 2750-2753 link
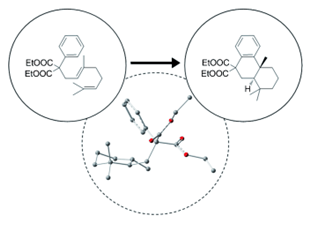
- Catalytic versatility and backups in enzyme active sites : The case of serum paraoxonase 1. Ben-David, M. ; Elias, M. ; Filippi, J. J. ; Dunach, E. ; Silman, I. ; Sussman, J. L. ; Tawfik, D.S. J. Molec Bio., 2012, 418, 181-196 link
- C-O and C-C bond formation in the cyclisation of gem-(dialkoxymethyl)-1,6-dienes catalysed by tin(IV) triflimidate at room temperature.
Vece, V. ; Ben, H. H. K. ; Antoniotti, S. ; Dunach, E. Tetrahedron Lett. 2012, 53 , 5102-5105 link
- Efficient Preparation of Anhydrous Metallic Triflates and Triflimides under Ultrasonic Activation. Legrave, N. ; Couhert, A. ; Olivero, S. ; Desmurs, J.-R. ; Dunach, E. Eur. J. Org. Chem. 2012, 901-904 link
- A Quantitative Approach of the Interaction between Metal Triflates and Organic Ligands Using Electrospray Mass Spectrometry. Gal, J.-F. ; Iacobucci, C. ; Monfardini, I. ; Massi, L. ; Dunach, E. ; Olivero, S. J. Am. Mass. Spec. 2012, 23, 2059-2062 link
![]() 2011_______________________________________________
2011_______________________________________________
- Biomimetic Cationic Polyannulation Reaction Catalyzed by Bi(OTf)3 : Cyclization of 1,6-Dienes, 1,6,10-Trienes, and Aryl Polyenes. Godeau, J. ; Olivero, S. ; Antoniotti, S. ; Dunach, E. Org. Lett. 2011, 13, 3320-3323 link
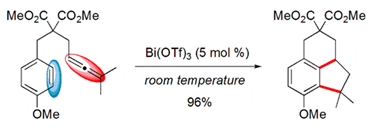
- Efficient Intramolecular Hydroarylation Catalysed by Bi(III) Triflate. Cacciuttolo, B. ; Poulain-Martini, S. ; Dunach, E. Eur. J. Org. Chem. 2011, 3710-3714 link
![]() 2010_______________________________________________
2010_______________________________________________
- In(III)-Catalysed Tandem C-C and C-O Bond Formation between Phenols and Allylic Acetates. Vece, V. ; Ricci, J. ; Poulain-Martini, S. ; Nava, P. ; Carissan, Y. ; Humbel, S. ; Dunach, E. Eur. J. Org. Chem. 2010, , 6239-6248 link
- An Opportunity for Mg-Catalyzed Grignard-Type Reactions : Direct Coupling of Benzylic Halides with Pinacolborane with 10 mol% of Magnesium. Pintaric, C. ; Olivero, S. ; Gimbert, Y. ; Chavant, P. Y. ; Dunach, E. J. Am. Chem. Soc. 2010, 132, 11825-11827 link
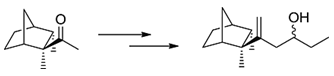
- Synthesis of New exo- and endo-3,8-Dihydro-β-santalols and Other Norbornyl-Derived Alcohols. Muratore, A. ; Clinet, J.-C. ; Dunach, E. Chem. Biodiversity 2010, 7, 623-638 link
- Mass spectrometric characterization of metal triflates and triflimides (Lewis superacid catalysts) by electrospray ionization and tandem mass spectrometry. Monfardini, I. ; Massi, L. ; Tremel, P. ; Hauville, A. ; Olivero, S. ; Dunach, E. ; Gal, J.-F. Rapid Commun. Mass Spectrom. 2010, 24 , 2611-2619 link
- Relative basicities toward metal triflates Lewis acids by electrospray mass spectrometry. Monfardini, I. ; Massi, L. ; Dunach, E. ; Olivero, S. ; Gal, J.-F. Chem. Commun. 2010, 46, 8472-8474 link
- Flavouring and odorant thiols from renewable natural resources by InIII-catalysed hydrothioacetylation and lipase-catalysed solvolysis. Dia, R.-M. ; Belaqziz, R. ; Romane, A. ; Antoniotti, S. ; Dunach, E. Tetrahedron Lett. 2010, 51, 2164-2167 link
- A convenient one pot preparation of 4-thiazolidinones from enaminolactones. Bouzroura, S. ; Bentarzi, Y. ; Kaoua, R. ; Nedjar-Kolli, B. ; Poulain-Martini, S. ; Dunach, E. Org. Commun. 2010, 3, 8-14
- N-Acyliminium ion chemistry : highly efficient and versatile carbon-carbon bond formation by nucleophilic substitution of hydroxy groups catalyzed by Sn(NTf(2))(4). Ben, O. R. ; Affani, R. ; Tranchant, M.-J. ; Antoniotti, S. ; Dalla, V. ; Dunach, E. Angew. Chem., Int. Ed. 2010, 49, 776-80 link
- Electrophilic functionalization of non-activated olefins catalyzed by Lewis superacids. Antoniotti, S. ; Poulain-Martini, S. ; Dunach, E. Synlett 2010, 2973-2988 link
- Metal triflimidates. Better than metal triflates as catalysts in organic synthesis. The effect of a highly delocalized counteranion. Antoniotti, S. ; Dalla, V. ; Dunach, E. Angew. Chem., Int. Ed. 2010, 49, 7860-7888 link
![]() 2009_______________________________________________
2009_______________________________________________
- Catalytic Friedel-Crafts allylation using Zn(II) triflimidate. Ricci, J. ; Poulain-Martini, S. ; Dunach, E. C. R. Chim. 2009, 12, 916-921 link
- Electrochemical preparation of pinacol allylboronic esters. Godeau, J. ; Pintaric, C. ; Olivero, S. ; Dunach, E. Electrochim. Acta 2009, 54, 5116-5119 link
- Lewis superacids derived from triflic and triflimidic acids and their use as catalysts in 1,6-diene cycloisomerization. Godeau, J. ; Olivero, S. ; Antoniotti, S. ; Dunach, E. C. R. Chim. 2009, 12, 911-915 link
- Radical-type reactions in protic and aprotic media : Comparisons in nickel-catalysed electrochemical reductive cyclizations. Dunach, E. ; Esteves, A. P. ; Medeiros, M. J. ; dos, S. N. C. S. ; Olivero, S. C. R. Chim. 2009, 12, 889-894 link
- Aluminium Triflate Catalysed Cyclisation of Unsaturated Alcohols : Novel Synthesis of Rose Oxide and Analogues. Coulombel, L. ; Weiwer, M. ; Dunach, E. Eur. J. Org. Chem. 2009, 5788-5795 link
- Tin(IV) triflimidate-catalyzed cyclization of epoxy esters to functionalized δ-hydroxy-γ-lactones. Antoniotti, S. ; Dunach, E. Tetrahedron Lett. 2009, 50, 2536-2539 link
![]() 2008_______________________________________________
2008_______________________________________________
- New norbornyl derivatives as woody fragrant materials. Muratore, A. ; Dunach, E. ; Clinet, J.-C. ; Plessis, C. Chem. Biodiversity 2008, 5, 1099-1114 link
- New aldehydes by catalytic diene cycloisomerisations. Grau, F. ; Bayon, J. C. ; Aguirre, P. A. ; Parella, T. ; Dunach, E. Eur. J. Org. Chem. 2008, 1214-1223 link
- Stereospecific cyclodehydration of 1,4-sulfanylalcohols to thiolanes : mechanistic insights. Filippi, J.-J. ; Dunach, E. ; Fernandez, X. ; Meierhenrich, U. J. Tetrahedron 2008, 64, 9999-10003 link
- Lewis super-acid catalyzed cyclizations : a new route to fragrance compounds. Coulombel, L. ; Grau, F. ; Weiwer, M. ; Favier, I. ; Chaminade, X. ; Heumann, A. ; Bayon, J. C. ; Aguirre, P. A. ; Dunach, E. Chem. Biodiversity 2008, 5, 1070-1082 link
- Aluminum triflate-catalyzed regioselective cycloisomerisation of non-activated unsaturated oximes. Chaminade, X. ; Chiba, S. ; Narasaka, K. ; Dunach, E. Tetrahedron Lett. 2008, 49, 2384-2387 link
- Reaction design for evaluation of the substrate range of hydrolases. Antoniotti, S. ; Fernandez, X. ; Dunach, E. Biocatal. Biotransform. 2008, 26, 228-234
- Facile preparation of metallic triflates and triflimidates by oxidative dissolution of metal powders. Antoniotti, S. ; Dunach, E., Chem. Commun. 2008, 993-995 link
![]() 2007_______________________________________________
2007_______________________________________________
- Indium triflate-catalysed addition of thio compounds to camphene : a novel route to 2,3,3-trimethyl-2-thiobicyclo[2.2.1]heptane derivatives. Weiewer, M. ; Chaminade, X. ; Bayon, J. C. ; Dunach, E. Eur. J. Org. Chem. 2007, 2464-2469 link
- Hydroalkoxylation of non-activated olefins catalyzed by Lewis superacids in alcoholic solvents : an eco-friendly reaction. Lemechko, P. ; Grau, F. ; Antoniotti, S. ; Dunach, E. Tetrahedron Lett. 2007, 48, 5731-5734 link
![]() 2006_______________________________________________
2006_______________________________________________
- Indium(III)-catalyzed highly regioselective addition of thiolacetic acid to non-activated olefins. Weiwer, M. ; Dunach, E. Tetrahedron Lett. 2006, 47 , 287-289 link
- Regioselective indium(III) trifluoromethanesulfonate-catalyzed hydrothiolation of non-activated olefins. Weiwer, M. ; Coulombel, L. ; Dunach, E. Chem. Commun. 2006, 332-334 link
- An environmentally friendly synthesis of functionalized indanes using electrochemical cyclization of ortho-halo-substituted homoallyl ethers and esters. Olivero, S. ; Perriot, R. ; Dunach, E. ; Baru, A. R. ; Bell, E. D. ; Mohan, R. S. Synlett 2006, 2021-2026 link
- Cycloisomerizaton of 1,6-dienes mediated by Lewis super acids without additives : easy access to polysubstituted six-membered carbocycles. Grau, F. ; Heumann, A. ; Dunach, E. Angew. Chem., Int. Ed. 2006, 45, 7285-7289 link
- Lewis acid-catalysed isomerization of thionolactones to thiolactones. Inversion of configuration. Filippi, J.-J. ; Fernandez, X. ; Dunach, E. Tetrahedron Lett. 2006, 47 , 6067-6070 link
- Intramolecular reductive cyclisations using electrochemistry : development of environmentally friendly synthetic methodologies. Dunach, E. ; Medeiros, M. J. ; Olivero, S., New J. Chem. 2006, 30, 1534-1548 link
- Aluminum(III) trifluoromethanesulfonate as an efficient catalyst for the intramolecular hydroalkoxylation of unactivated olefins : experimental and theoretical approaches. Coulombel, L. ; Rajzmann, M. ; Pons, J.-M. ; Olivero, S. ; Dunach, E. Chem.Eur. J. 2006, 12, 6356-6365 link
- Novel catalytic tandem isomerization/cyclization reaction of α-methallyloxy carboxylic acids. Chaminade, X. ; Coulombel, L. ; Olivero, S. ; Dunach, E. Eur. J. Org. Chem. 2006, 3554-3557 link
- Density functional theory investigations on acid-catalyzed epoxide oxidative ring-opening by DMSO. Competition between oxidation processes. Antoniotti, S. ; Golebiowski, J. ; Cabrol-Bass, D. ; Dunach, E. J. Mol. Struct. : THEOCHEM 2006, 763, 155-159 link
![]() 2005_______________________________________________
2005_______________________________________________
- Conversion of norbornene derivatives into vicinal-dithioethers via S8 activation. Poulain, S. ; Julien, S. ; Dunach, E. Tetrahedron Lett. 2005, 46 , 7077-7079 link
- Electrochemical intramolecular cyclization of propargyl bromoethers catalyzed by nickel complexes. Dunach, E. ; Paula, E. A. ; Medeiros, M. J. ; Olivero, S. New J. Chem. 2005, 29, 633-636 link
- Catalytic formation of C-O bonds by alkene activation. Lewis acid-cycloisomerisation of olefinic alcohols. Coulombel, L. ; Favier, I. ; Dunach, E. Chem. Commun. 2005, 2286-2288 link
- Cycloisomerization of olefinic carboxylic acids catalyzed by trifluoromethanesulfonic acid. Coulombel, L. ; Dunach, E., Synth. Commun. 2005, 35, 153-160 link
![]() 2004_______________________________________________
2004_______________________________________________
- Nickel-catalyzed electrochemical synthesis of dihydro-benzo[b]thiophene derivatives. Pelletier, J. ; Olivero, S. ; Dunach, E. Synth. Commun. 2004, 34, 3343-3348 link
- Electrosynthesis of dihydrobenzo[b]thiophene derivatives catalyzed by nickel complexes. Pelletier, J. ; Olivero, S. ; Dunach, E. Proc. - Electrochem. Soc. 2004, 10, 129-132
- CoCl2 catalyzed decarboxylation-oxidation of mandelic acids by molecular oxygen. Favier, I. ; Dunach, E. ; Hebrault, D. ; Desmurs, J.-R. New J. Chem. 2004 , 28 , 62-66 link
- New protic salts of aprotic polar solvents. Favier, I. ; Dunach, E. Tetrahedron Lett. 2004, 45, 3393-3395 link
- Triflic acid-catalyzed cyclization of unsaturated alcohols. Coulombel, L. ; Dunach, E. Green Chem. 2004, 6, 499-501 link
- Structural studies of mono- and dimetallic MoVI complexes - a new mechanistic contribution in catalytic olefin epoxidation provided by oxazoline ligands. Brito, J. A. ; Gomez, M. ; Muller, G. ; Teruel, H. ; Clinet, J.-C. ; Dunach, E. ; Maestro, M. A. Eur. J. Inorg. Chem. 2004, 4278-4285 link
- Studies on the catalytic oxidation of epoxides to α-diketones by Bi(0)/O2 in DMSO. Antoniotti, S. ; Dunach, E. J. Mol. Catal. A : Chem. 2004, 208, 135-145 link
- Recent uses of bismuth derivatives in organic synthesis. Antoniotti, S. ; Dunach, E. C. R. Chim. 2004, 7, 679-688 link
- Mechanistic aspects of the bismuth-catalysed oxidation of epoxides to α-diketones. Antoniotti, S. ; Dunach, E. Eur. J. Org. Chem. 2004,3459-3464 link
- Catalytic epoxide oxidation : a novel access to flavouring and odorant α-diketones. Antoniotti, S. ; Alezra, N. ; Fernandez, X. ; Dunach, E. Flavour Fragrance J. 2004, 19, 373-381 link
![]() 2003_______________________________________________
2003_______________________________________________
- New electrosynthesis of arylboronic esters from aromatic halides and pinacolborane. Laza, C. ; Dunach, E. Adv. Synth. Catal. 2003, 580-583 link
- Novel method for synthesis of arylboronic acids and esters by electroreduction of halogenated aromatic derivatives in the presence of borating agents. Laza, C. ; Dunach, E. C. R. Chim. 2003, 6, 185-187 link
- Novel electrosynthesis of arylboronic acids and esters. Laza, C. ; Dunach, E. Proc. - Electrochem. Soc. 2003, 12, 21-24
- α,β-Unsaturated 1,3-oxathiolanes as masked heterodienes in the thio Diels-Alder reaction with styrene derivatives. Kerverdo, S. ; Lizzani-Cuvelier, L. ; Dunach, E. Tetrahedron Lett. 2003, 44, 853-856 link
- Thio Diels-Alder reactions of α,β-unsaturated 1,3-oxathiolanes with aliphatic olefins and 1,3-dienes. Kerverdo, S. ; Lizzani-Cuvelier, L. ; Dunach, E. Tetrahedron Lett. 2003, 44, 8841-8844 link
- Novel electrosynthesis of metallic bis(trifluoromethanesulfonyl) imides. Favier, I. ; Dunach, E. Tetrahedron Lett.2003, 44, 2031-2032 link
- Oxidation of mandelic acid derivatives catalyzed by Bi(0)/O2 systems : mechanistic considerations. Favier, I. ; Dunach, E. Tetrahedron 2003, 59, 1823-1830 link
- Carbon-carbon bond formation with electrogenerated nickel and palladium complexes. Dunach, E. ; Franco, D. ; Olivero, S. Eur. J. Org. Chem. 2003, 1605-1622 link
- Epoxide oxidations. A valuable tool in organic synthesis. Antoniotti, S. ; Dunach, E. Synthesis 2003, 2753-2762 link
![]() Brevets_____________________________________________
Brevets_____________________________________________
- Nouveaux polymères contenant des sels de lithium ou de sodium de bis(sulfonyl)imides greffes, leurs procedes de preparation et leurs utilisations comme electrolytes pour batteries. V. Morizur, S. Olivero, P. Knauth, J. R. Desmurs, E. Dunach, CDP-Innovation ; PCT WO2016/012670 du 28/01/2016. Demande de brevet aux Etats-Unis numéro US15/327,606 déposée le 19/01/2017 ; publiée le : 15/06/2017 sous le numéro US20170170516A1
- Nouveaux polymères contenant des sels de lithium ou de sodium de sulfonamides, leurs procedes de preparation et leurs utilisations comme electrolytes pour batteries. Polymers containing lithium or sodium sulfonamide salts, their methods of preparation and their uses as electrolytes for batteries. V. Morizur, S. Olivero, J. R. Desmurs, E. Dunach, CDP-Innovation ; PCT WO2016/012669 du 28/01/2016. Demande de brevet aux Etats-Unis numéro US15/327,609 déposée le 19/01/2017 ; publiée le :22/06/2017 sous le numéro US20170179526A1
- Nouveaux polymères contenant des fonctions sulfonates métalliques, leur procédés de préparation et leurs utilisation comme antibactériens, fongicides et antimicrobiens. V. Morizur, S. Olivero, A. Adao, J.R. Desmurs, E. Duñach. FR 3030534B1 du 12/05/2017.
- Nouveaux matériaux à effets optiques et leurs applications. V. Morizur, S. Olivero, J.R. Desmurs, E. Duñach, Fr Dépôt Fév. 2017.
- Nouveaux polymères contenant des sels de lithium ou de sodium de bis(sulfonyl)imides greffes, leurs procedes de preparation et leurs utilisations comme electrolytes pour batteries V. Morizur, S. Olivero, P. Knauth, J. R. Desmurs, E. Dunach, CDP-Innovation ; Appl. FR N° 2013-59771 ; FR Pat. N° 3007647A1 2015.
- Nouveaux polymères contenant des sels de lithium ou de sodium de sulfonamides, leurs procedes de preparation et leurs utilisations comme electrolytes pour batteries V. Morizur, S. Olivero, J. R. Desmurs, E. Dunach, CDP-Innovation ; Demande de brevet français, 2014, n° 14/01709, 2014.
- Nouveaux polymères contenant des fonctions sulfonates d’ammonium, leur procédés de préparation et leurs utilisation comme catalyseurs, antibactériens, fongicides V. Morizur, S. Olivero, J.R. Desmurs, E. Duñach, Fr Dépôt 14/02989, 2014.
- Nouveaux polymères contenant des fonctions sulfonates métalliques, leur procédés de préparation et leurs utilisation comme antibactériens, fongicides et antimicrobiens V. Morizur, S. Olivero, A. Adao, J.R. Desmurs, E. Duñach, Fr Dépôt 14/02990, 2014.
- Nouveaux polymères contenant des fonctions sulfonates métalliques, leur procédés de préparation et leurs utilisation comme catalyseurs V. Morizur, S. Olivero, J.R. Desmurs, E. Duñach, Fr Dépôt 14/02991, 2014.
- Alcool Polyvinylique Fonctionnalise par un dérivé Aromatique et/ou un dérivé Aliphatique B. Boulay, E. Delattre, J. R. Desmurs, E. Dunach, G. Lemière 2013, french patent submission number 13 59771.
- Preparation of metal salts of triflic acid and of triflimidic acid by ultrasonic treatment. Desmurs, Jean Roger ; Dunach, Isabel ; Olivero, Sandra ; Antoniotti, Sylvain. PCT Int. Appl. 2012, WO 2012010752 A1 20120126.
- Procédé de preparation d’acides et d’esters boroniques en presence de magnesium métallique. E. Dunach, S. Olivero, C. Pintaric, PCT Int. Appl. 2010, WO 2010055245 A2 20100520.
- Isopropylmethylcyclopentane . J. Mane, E. Dunach, A. Muratore, J. C. Clinet, FR 09 53707, 2009.
- Nouveaux aldéhydes à structure norbornane : préparations et utilisations en parfumerie. J. Mane, A. Muratore, I. Dunach, J. C. Clinet. 2008, PCT/WO 2009/138657 A1.
- Procédé de préparation de de la 5,5-dimethyl-3-ethyl-3,4-dihydrofuran-2-one. Mane, J. M. E. ; Clinet, J. C. ; Clinet, I. ; Colombel, L. ; Marin, C. FR 2 906 531 A1 2007.
- Utilisation en parfumerie et aromatique et procédé de préparation de la la 5,5-dimethyl-3-ethyl-3,4-dihydrofuran-2-one. J. Mane, J. C. Clinet, I. Dunach, L. Coulombel, C. Marin, V Mane Fils, Fr. ; PTC/FR 200705 2004.
- Method for transforming a 1,2-disubstituted epoxide cycle into its alpha-diketone derivative. I. Dunach-Clinet, S. Antoniotti (Rhodia Chimie, Fr.), PCT/WO 03/022789 A2, 2003.